Tamil Nadu manufacturer was arrested recently
TNR News Network
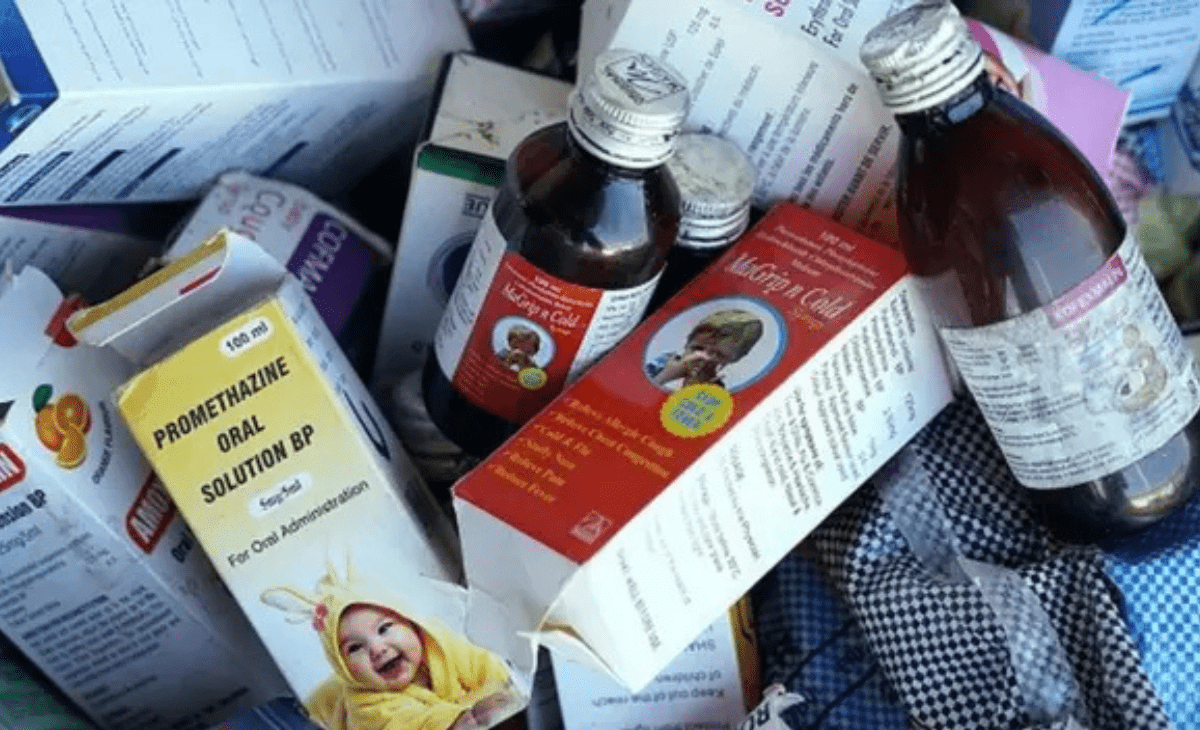
Shimla: In the wake of recent d*eaths in Madhya Pradesh and Rajasthan allegedly caused by toxic cough syrups, the Central Drugs Standard Control Organisation (CDSCO) has flagged two cough syrup samples from Himachal Pradesh as “not of standard quality” while one was declared adulterated.
The alert comes even as authorities in Tamil Nadu recently arrested the owner of a pharmaceutical firm accused of manufacturing a deadly cough syrup.
According to the CDSCO’s September drug alert, as many as 112 drug samples manufactured across the country failed quality tests, including medicines for heart disease, cancer, diabetes, hypertension, asthma, infections, pain, inflammation, anaemia and epilepsy.
Among these, three cough syrups — two produced in Sirmaur, Himachal Pradesh, and one in Haridwar, Uttarakhand — were found unsafe, with one identified as fake.
Officials clarified that the failure pertains to specific batches only and the alert did not imply that all products from the same companies are substandard. However, the findings have sparked serious concern, especially after dozens of children reportedly died in Madhya Pradesh and Rajasthan due to consumption of contaminated cough syrups earlier this year.
The adulterated sample, reportedly marketed under the label Besto-Cough Dry Cough Formula, is under detailed scrutiny, with authorities verifying the manufacturer’s identity and address.
Of the total failed samples, 49 were from Himachal Pradesh, followed by 16 from Gujarat, 12 from Uttarakhand and 11 from Punjab. Six samples from Madhya Pradesh and several others from Sikkim, Telangana, Andhra Pradesh, Karnataka, Maharashtra, West Bengal, Rajasthan, Uttar Pradesh, Haryana, and Jammu & Kashmir were also found substandard.
The latest alert has once again brought the spotlight on Himachal Pradesh’s pharmaceutical sector, often called the “drug manufacturing hub of India”, raising questions over the quality control mechanisms in place.
Health officials have advised consumers to check batch numbers carefully and avoid purchasing medicines from unverified sources as investigations into the failed samples continue.